Publications
Published Research Articles
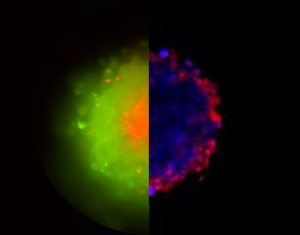
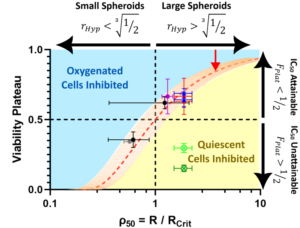
Hypoxia-Induced Drug-Resistant Bias 3D Cancer Spheroid Drug Screens
APL Bioengineering (2026) [IN REVIEW]
Tiger H. Shi, Yu-Tang Huang, Hyunsu Jeon, Daniel Montes-Pinzon, Peter Mu-Hsin Chang, Nai-Jung Chiang, John Alex Sinclair, Angela Taglione, Donny Hanjaya-Putra, Yichun Wang, Chi-Ying F. Huang, and Hsueh-Chia Chang
Cellular 3D cancer spheroid technologies are novel tools that facilitate large-scale drug screening to bridge the in vitro-in vivo gap, without the cross-species effects of animal models. However, many spheroid studies fail to achieve IC50 (dosage for 50% inhibition) even for unreasonably high applied drug concentrations (up to 1000× 2D IC50). By mapping oxygen transport in patient-derived pancreatic cancer spheroids, this limiting viability is attributed to a near-universal oxygen decay gradient that renders cells deeper than 20 µm from the spheroid surface hypoxically quiescent and resistant to many chemotherapeutic drugs. The dose-independent viability barrier prevents IC50 from being achieved for spheroids larger than 150 µm in diameter, if the applied drug is dependent on the proliferating cell behavior. By examining 3 cancer cell types and 5 chemotherapeutic drugs, targeting this limiting viability barrier allows the selection of drugs and adjuvants that are effective in treating all cell populations within a spheroid. The reported analysis provides a framework for the accurate assessment of drug efficacy to target both well-oxygenated proliferating cells and hypoxically quiescent cells in biologically relevant and realistic 3D spheroid systems.
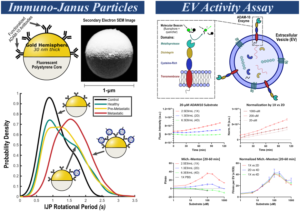
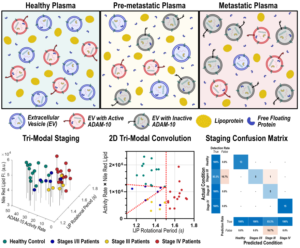
Multimodal Activity-Affinity Assay of ADAM-10 Extracellular Vesicles in Untreated Plasma Reveals Metastatic Stage of Colorectal Cancer
Biosensors and bioelectronics (2026) [IN PRESS]
Tiger Shi‡ & J. Alex Sinclair‡, Youwen Zhang, Xuemin Lu, Sonu Kumar, Xin Lu, Satyajyoti Senapati, and Hsueh-Chia Chang
Metalloproteinases (MPs) such as a-disintegrin and metalloproteinase-10 (ADAM-10) are key drivers of extracellular matrix remodeling during tumor progression, yet MP-based liquid biopsy tests have not reached clinical utility. Here, we show that active ADAM-10 is selectively enriched on the surface of circulating extracellular vesicles (EVs) in the plasma of colorectal cancer patients. Our findings further suggest ADAM-10+ EVs are locally enriched in dense pre-metastatic tumor extracellular matrix and subsequently accumulate in blood post-metastasis. To capture these unique signatures of disease progression, a novel ADAM-10 activity assay is integrated with a size-selective Immuno-Janus Particle (IJP) affinity assay for characterizing ADAM-10+ EVs in untreated plasma. In a 43-patient colorectal cancer cohort, this multimodal platform distinguished healthy, pre-metastatic, and metastatic states with 95% overall accuracy. When combined with lipidomics as a third modality, the platform correctly determined 97.4% cancer stage accuracy, with only one misclassification. This study establishes a multimodal EV-based activity/affinity assay as a robust framework for liquid biopsy, providing accurate cancer staging, improved prognostics, and offering a potential platform for pan-disease diagnostics.

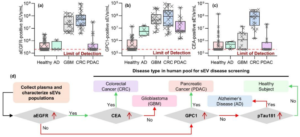
Immunojanus Particles for low-volume and isolation-free unlabeled characterization of small Extracellular Vesicle in biofluids: Characterization of disease type by surface marker profiling
Nature Biomedical Engineering (2026) [IN PRESS]
Sonu Kumar‡ & John Alex Sinclair‡, Tiger Shi, Han-Sheng Chuang, Satyajyoti Senapati, and Hsueh-Chia Chang
Small extracellular vesicles (sEVs) are vital for cellular communication and serve as critical biomarker carriers for diseases such as cancer. However, quantifying and profiling sEV surface markers presents significant challenges due to the low concentration of specific sEV-bound proteins and interference by more abundant dispersed proteins. This paper presents Immunojanus Particles (IJPs), a new method that enables the direct detection of sEVs in less than an hour without isolation. The design of IJPs incorporates fluorescent and non fluorescent halves, utilizing rotational Brownian motion to detect captured sEVs through the change in the blinking rate, without interference from the smaller dispersed proteins. We demonstrate a detection limit of 2E5 sEVs/mL with low sample volumes and the capability to characterize sEVs directly from plasma, serum, cell culture media, and urine. In a small pilot study involving 87 subjects, including individuals with colorectal cancer, pancreatic ductal adenocarcinoma, glioblastoma, Alzheimer’s disease, and healthy controls, our method accurately identified the type of disease with a high 0.90-0.99 AUC in a blind setting. Compared with an orthogonal ultracentrifugation plus surface plasmon resonance (UC+SPR) method that requires about 24 hours, the sensitivity and dynamic range of IJP are better by 2 logs.
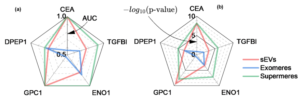
Surface Markers on Supermeres Outperform Extracellular Vesicles in Colorectal Cancer Diagnosis
Nature Scientific Reports (2026)
Sonu Kumar, John Alex Sinclair, Tiger Shi, Gauen Kim, Runyao Zhu, Grace Gasper, Yichun Wang, James N. Higginbotham, Qin Zhang, Dennis K. Jeppesen, Oleg Tutanov, Maxwell Hamilton, Jeffrey L. Franklin, Al Charest, Robert Coffey, Satyajyoti Senapati & Hsueh-Chia Chang
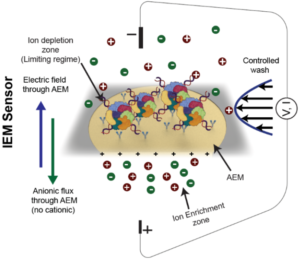
Extracellular nanocarriers in biofluids, including blood, urine, and cell culture media, comprise diverse lipid–protein–nucleic acid assemblies such as extracellular vesicles (EVs), lipoproteins, and non-membranous particles like supermeres and exomeres. Among these, supermeres, hold significant diagnostic potential but are challenging to characterize due to (a) lack of information on their surface composition and (b) labor-intensive isolation methods requiring large starting volumes and days of serial ultracentrifugation. This study introduces a novel approach for supermere characterization using an Ion Exchange Membrane Sensor (IEMS), enabling rapid, isolation-free detection within 30 minutes from 50 μL of sample, with a sensitivity of 1E6–1E7 supermeres/mL. Orthogonal validation through ultracentrifugation and surface plasmon resonance (UC+SPR) confirmed the robustness and specificity of IEMS. Supermeres are enriched with RNA, contributing to a pronounced negative zeta potential, and contain key proteins such as HSPA13, ENO2, and DDR1, which are composition-wise analogous to tetraspanins in small extracellular vesicles (sEVs). Importantly, supermeres outperform sEVs and exomeres across multiple shared and unique surface proteins critical to colorectal cancer diagnostics, demonstrating significantly greater diagnostic utility. Notably, substantial decreases in supermere concentrations were observed post-resection surgery, further underscoring their clinical relevance. This study highlights the potential of supermeres to surpass existing EV-based biomarkers in precision medicine applications.
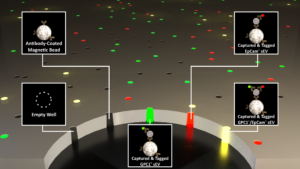
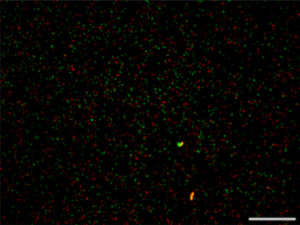
A Mem-dELISA platform for dual color and ultrasensitive digital detection of colocalized proteins on extracellular vesicles
Biosensors and Bioelectronics (2025), 267, 116848
Himani Sharma‡ & Vivek Yadav‡, Alice Burchett, Tiger Shi, Satyajyoti Senapati, Meenal Datta, Hsueh-Chia Chang
Accurate, multiplex, and ultrasensitive measurement of different colocalized protein markers on individual tumor-derived extracellular vesicles (EVs) and dimerized proteins with multiple epitopes could provide insights into cancer heterogeneity, therapy management and early diagnostics that cannot be extracted from bulk methods. However, current digital protein assays lack certain features to enable robust colocalization, including multi-color detection capability, large dynamic range, and selectivity against background proteins. Here, we report a lithography-free, inexpensive (< $0.1) and ultrasensitive dual-color Membrane Digital ELISA (MemdELISA) platform by using track-etched polycarbonate (PCTE) membranes to overcome these shortcomings. Their through-pores remove air bubbles through wicking before they are sealed on one side by adhesion to form microwells. Immunomagnetic bead-analyte complexes and substrate solution are then loaded into the microwells from the opposite side, with >80% loading efficiency, before sealing with oil. This enables duplex digital protein colorimetric assay with beta galactosidase and alkaline phosphatase enzymes. The platform achieves 5 logs of dynamic range with a limit of detection of 10 aM for both Biotinylated β-galactosidase (B-βG) and Biotin Alkaline Phosphatase Conjugated (B-ALP) proteins. We demonstrate its potential by showing that a higher dosage of paclitaxel suppresses EpCAM-positive EVs but not GPC-1 positive EVs from breast cancer cells, a decline in chemo-resistance that cannot be detected with Western blot analysis of cell lysate. The Mem-dELISA is poised to empower researchers to conduct ultrasensitive, high throughput protein colocalization studies for disease diagnostics, treatment monitoring and biomarker discovery.
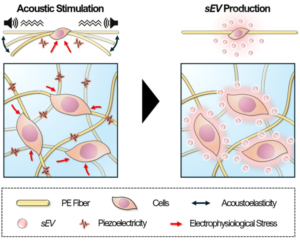
Stimulative piezoelectric nanofibrous scaffolds for enhanced small extracellular vesicle production in 3D cultures
Biomaterials Science (2024), 12 (22), 5728–5741
James Johnston‡ & Hyunsu Jeon‡, Yun Young Choi, Gaeun Kim, Tiger Shi, Courtney Khong, Hsueh-Chia Chang, Nosang Vincent Myung, and Yichun Wang
Small extracellular vesicles (sEVs) have great promise as effective carriers for drug delivery. However, the challenges associated with the efficient production of sEVs hinder their clinical applications. Herein, we report a stimulative 3D culture platform for enhanced sEV production. The proposed platform consists of a piezoelectric nanofibrous scaffold (PES) coupled with acoustic stimulation to enhance sEV production of cells in a 3D biomimetic microenvironment. Combining cell stimulation with a 3D culture platform in this stimulative PES enables a 15.7-fold increase in the production rate per cell with minimal deviations in particle size and protein composition compared with standard 2D cultures. We find that the enhanced sEV production is attributable to the activation and upregulation of crucial sEV production steps through the synergistic effect of stimulation and the 3D microenvironment. Moreover, changes in cell morphology lead to cytoskeleton redistribution through cell–matrix interactions in the 3D cultures. This in turn facilitates intracellular EV trafficking, which impacts the production rate. Overall, our work provides a promising 3D cell culture platform based on piezoelectric biomaterials for enhanced sEV production. This platform is expected to accelerate the potential use of sEVs for drug delivery and broad biomedical applications.
In-Progress Articles
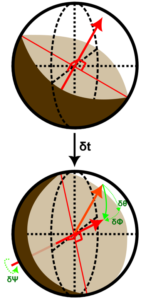
Theoretical Study of Janus Particle Rotational Behavior
TBD
Tiger H. Shi, J. Alex Sinclair, & Hsueh-Chia Chang
Using stochastic calculus and probability theory, we interrogate the rotational dynamics of the Immuno-Janus Particle (IJP) technology. Doing so reinforces the efficacy of the IJP platform as a diagnostic system and illuminates the design space to enhance the lab-work protocol and analytical software.

An In Situ stimulative perfusion bioreactor for large scale production of therapeutic small extracellular vesicles
TBD
James Johnston & Tiger H. Shi, Simon Yan, Hsueh-Chia Chang, and Yichun Wang
Scale-up of previously developed piezoelectric bioscaffolding into a large-volume, perfusion flow, semi-batch bioreactor to generate high concentration sEVs for therapeutic, cosmetic, and biological discovery applications. Currently, testing the developed bioreactor unit with various cell lines and characterizing effluent sEV populations given flow and acoustic stimulation.

Large-Scale, Rapid Immersed AC Electrospray (iACE) Droplet Generation
TBD
Tiger H. Shi, Sinyu Chen, Chi-ying F. Huang, and hsueh-chia chang
Adapting several earlier technologies from the Chang group. Droplets <20-μm can be generated rapidly at ~1E6 droplets per second. At such speeds, we will be able to use them for applications ranging from biosensing to novel therapeutics.